(Source: Karlsruhe Institute of Technology [5952])
DieselNet Technology Guide » Methane Emission Control Catalysts
DieselNet | Copyright © ECOpoint Inc. | Revision 2024.10
This is a preview of the paper, limited to some initial content. Full access requires DieselNet subscription.
Please log in to view the complete version of this paper.
The oxidation of methane over an oxidation catalyst is described by the equation:
CH4 + 2O2 = CO2 + 2H2O(1)
Since oxygen is needed, methane oxidation catalysts (MOC) can only be used to control CH4 emissions from lean-burn gas engines. In stoichiometric (sometimes referred to as “rich burn”) gas engines, methane can be controlled with a three-way catalyst (TWC).
Methane is a highly stable compound—high activation energy is required to break the C-H bond and to oxidize the molecule. Therefore, a higher catalyst temperature is necessary for the oxidation of methane, compared to longer chain hydrocarbons. To achieve high CH4 conversions under realistic conditions, methane oxidation catalysts must be operated at temperatures of around 500°C. While some laboratory studies reported CH4 catalyst light-offs (T50) of less than 350°C [3666], such results are limited to catalysts of very high precious metal loadings, tested without or with very low exposure to sulfur.
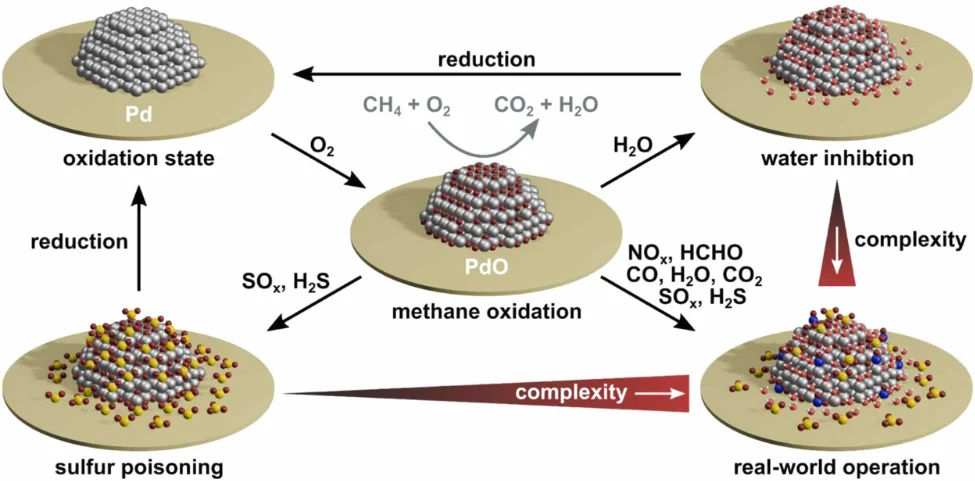
Pd-based catalysts are generally considered to be most active to oxidize methane and other short chain hydrocarbons, Figure 1 [3651].
CH4 1000 ppm, O2 10%, H2O 10%, SV = 40,000 1/h.
However, in addition to the high catalyst temperature required, palladium catalysts face other challenges. These include, Figure 2:

(Source: Karlsruhe Institute of Technology [5952])
Other precious metals considered for methane oxidation are platinum and rhodium. Platinum-based catalysts are of interest for lean-burn applications due to their low sensitivity to water and sulfur containing compounds. Platinum does not form stable hydroxides or sulfates. Sulfur can even promote methane oxidation over platinum supported catalysts. However, the low temperature activity of platinum-based catalysts for methane oxidation is poor under lean conditions, Figure 1 [3649][3651]. Oxidation catalysts based on Rh have also been tested. Rhodium has light-off characteristics between those for Pd and Pt. Its sensitivity to sulfur poisoning also falls between that for Pd and Pt [3650].
One concept proposed to enhance the catalyst performance is a pre-turbine catalyst location, where the exhaust gas stream exhibits higher temperatures and where higher pressure can be utilized for pollutant conversion [6194]. High pressure results in a higher density and consequently in a lower space velocity that increases the catalyst residence time of pollutants. However, catalyst placement upstream the turbocharger also results in a higher partial pressure of water, which enhances the inhibitory effect of steam over Pd-based catalysts. Additionally, the upstream placement of the catalyst may be a source of turbocharger response problems.
###