(Courtesy of LEC GmbH)
DieselNet | Copyright © ECOpoint Inc. | Revision 2025.07a
This is a preview of the paper, limited to some initial content. Full access requires DieselNet subscription.
Please log in to view the complete version of this paper.
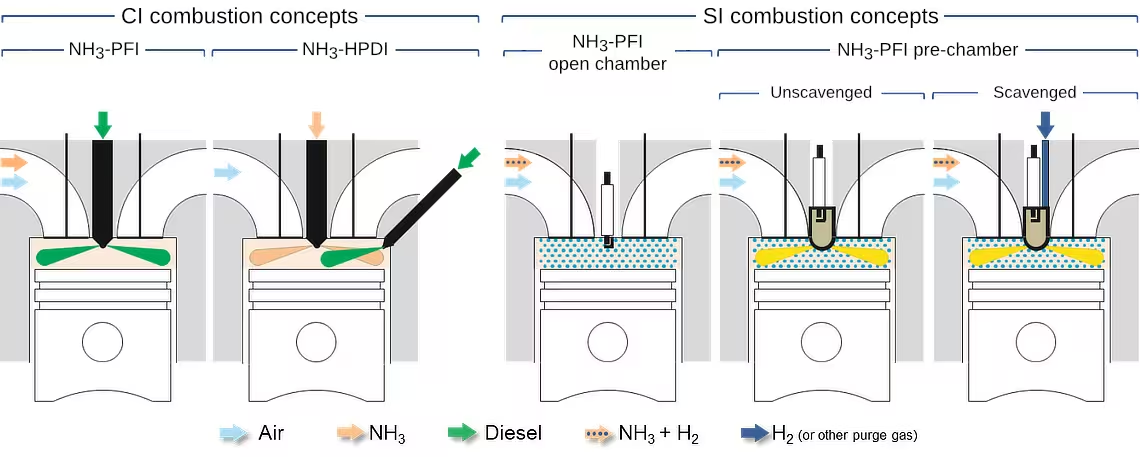
As an engine fuel, ammonia has seen sporadic use for well over a century [5368]. Due to its high auto-ignition temperature, high heat of vaporization, high minimum ignition energy and narrow flammability range, a dual fuel CI strategy such as ignition with a diesel pilot is typically used, Figure 1. While SI strategies are also possible, hydrogen is typically added to ammonia to facilitate ignition. This hydrogen can be supplied as an external gas stream or else it can be generated through ammonia decomposition. Spark ignition of neat ammonia is possible under some conditions.

(Courtesy of LEC GmbH)
As is the case with natural gas engines, ammonia engines can operate on different cycles depending on how fuel is introduced and ignition is achieved. While spark ignition engines in Figure 1 operate on the Otto cycle, the dual fuel engines can represent Otto cycle, diesel cycle or a dual fuel mixed cycle depending on when and how ammonia is introduced and the quantity of the pilot fuel injected. Premixing ammonia with air in the port or an early direct injection and using a relatively small pilot injection for ignition would represent Otto cycle operation while ignition with a large pilot would be dual fuel mixed cycle operation. Late direct injection of ammonia ignited by a diesel pilot would represent diesel cycle operation.
While there are no direct carbon emissions from ammonia combustion, the use of hydrocarbons as the pilot fuel would result in CO2 emissions. Lifecycle GHG emissions from ammonia depend strongly on feedstock and manufacturing process details.
Emissions of unburned ammonia and N2O are a concern with ammonia fueled engines and levels in the exhaust could potentially be several times higher than engines burning conventional fuels. Ammonia emissions are a concern from a toxicity perspective while N2O is a powerful greenhouse gas [5367]. Emission levels of 1.9 g/kWh of N2O would have the same climate warming effect as CO2 emissions from diesel combustion [5728].
Emissions of unburned ammonia and N2O are significant in Otto cycle and dual fuel mixed cycle operation where ammonia and air are premixed well before combustion. One contributor to this challenge is the presence of combustion chamber crevices. Ammonia trapped in crevices during combustion and released during expansion is a significant source of unburned ammonia. It may also be an important source of N2O emissions as the unburned ammonia released from the crevices is only partially oxidized [5915]. Flame quenching near combustion chamber surfaces is another potential source of unburned ammonia.
Diesel cycle operation shows the potential of low-speed two-stroke dual fuel engines using late cycle injection of ammonia to have low emissions of ammonia and N2O. However, ammonia leakage from the fuel injector can be a significant source of unburned ammonia but can be mitigated with suitable injector design [6462][6471].
The emission challenges of using ammonia as a fuel in Otto cycle and dual fuel mixed cycle engines is apparent in a number of studies in which a dual fuel approach is used with a premixed ammonia charge and a diesel pilot for ignition [5395][4672][5230][5370][5396][5716][6477]. These studies were carried out with diesel engines ranging in size from about 1 L/cylinder to 2.4 L/cylinder and in all of them, ammonia was introduced into the intake manifold which allowed the ammonia and air to premix. While increasing ammonia fraction tended to decrease NOx emissions, in studies that also measured N2O emissions, the lower NOx emissions were often associated with increased N2O emissions. N2O is produced almost entirely from the decomposition of ammonia. Unburned ammonia emissions also tended to be high with values as high as 35 g/kWh and up to 15% of the supplied ammonia escaping combustion. Advancing the diesel injection timing typically resulted in lower N2O emissions and improved thermal efficiency but increased NOx emissions. Richer ammonia air ratios also lower N2O emissions.
Considering GHG emissions, safety issues with ammonia and other criteria, the Mærsk Mc-Kinney Møller shipping industry consortium proposed emission target levels of 10-30 ppm for NH3 and 0.06 g/kWh for N2O [5728].
Ammonia engines are also capable of producing particulate emissions not only from lubricating oil but from other sources as well. Sulfate from sulfur in lubricating oil and nitrate salts with ammonium have been identified as potential sources of engine-out particulate emissions [6522][6521]. If an SCR catalyst is used, ammonium nitrate and urea derived particles can also be formed in the aftertreatment system.
An optimal—albeit not yet developed—aftertreatment system that has been suggested to achieve low emission levels is an SCR catalyst optimized to reduce both NOx and N2O, followed by an NH3 slip catalyst [5728]. The ammonia that escapes combustion in the engine could contribute to NOx reduction over the SCR catalyst but depending on the NH3/NOx ratio, further urea or ammonia injection upstream of the SCR catalyst may be required to achieve NH3/NOx ~1 required to maximize NOx reduction.
Ammonia slip catalysts are currently used in many diesel applications equipped with an SCR system for NOx reduction. They could also be applied to ammonia engines downstream of an SCR catalyst. Ammonia oxidation activity on these catalysts is often a trade-off with selectivity towards NOx and N2O—i.e., catalysts with a higher ammonia oxidation activity and lower light-off temperature tend to oxidize the ammonia to NOx and N2O instead of N2. Thus, these catalysts tend to have lower precious metal loadings (usually Pt) and a higher light-off temperature (around 250°C) to ensure ammonia is oxidized to N2. A dual layer catalyst with an SCR catalyst layer over the oxidation catalyst is commonly used to further improve selectivity towards N2.
In cases where the unburned ammonia emissions from the engine are too high for the SCR/ASC combination, an ammonia oxidation catalyst (AMOX) placed upstream of the SCR catalyst could be used. Ammonia is partly oxidized on the AMOX (e.g., a Pt/Pd oxidation catalyst) to NOx, which reacts with NH3 in the SCR catalyst [6519].
In some applications, N2O decomposition catalysts may be required. Unlike N2O reduction on an SCR catalyst, N2O decomposition catalysts do not require a reductant. Decomposition of N2O can be achieved with noble metal catalysts, non-precious metal oxides, and molecular sieve catalysts [6513][6514][6515][6516].
###