(Adapted from [5197])
DieselNet Technology Guide » Powertrain Alternatives
DieselNet | Copyright © ECOpoint Inc. | Revision 2025.12
This is a preview of the paper, limited to some initial content. Full access requires DieselNet subscription.
Please log in to view the complete version of this paper.
The battery is arguably the most critical component of a battery electric vehicle (BEV) and a very important component of hybrid and plug-in hybrid vehicles. With the increased focus on improving the driving range and reducing costs of electrified vehicles, battery technology has seen significant advances since the 1990s. Much of the focus has been on improving battery capacity and safety while minimizing increases in mass and reducing cost.
Li-ion batteries are the dominant type of battery for vehicle applications. Many variations of Li-ion batteries are possible depending on the electrode and electrolyte materials selected.
Figure 1 shows some details of a typical Li-ion battery cell. The main components are a graphite anode, a lithium metal oxide cathode and an electrolyte. The cathode in this illustration is lithium cobalt oxide (LCO), LiCoO2, but other cathode materials are possible as well. The anode incorporates a copper current collector and the cathode an aluminum current collector. In this example, a liquid electrolyte of a lithium salt and an organic solvent are used (e.g., LiPF6 salt with ethylene carbonate electrolyte). A separator, such as a microporous polymer membrane (e.g., polyolefin resins such as polyethylene (PE), polypropylene (PP) or their composites are common), prevents physical contact of the electrodes while allowing the exchange of lithium ions between the electrodes. A number of cells are packaged together to form a battery pack.
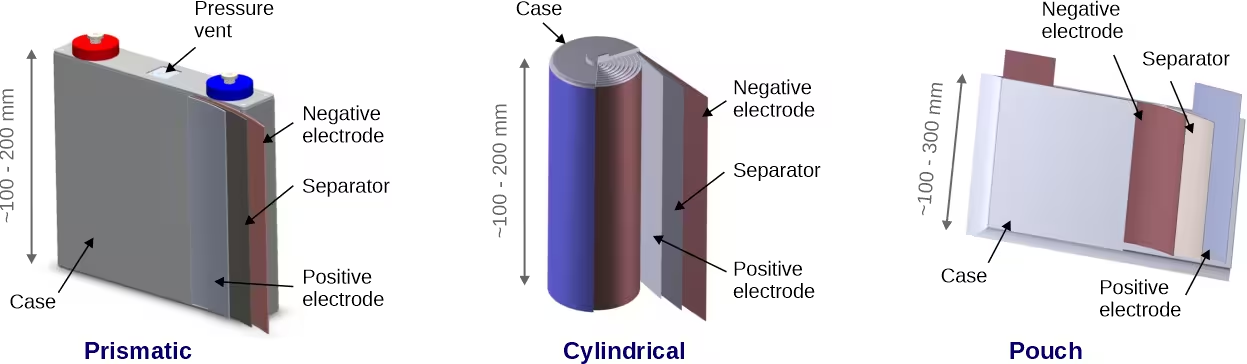
In practice, the components can be manufactured as thin foils that can be packaged into different configurations such as the cylindrical, pouch and prismatic geometries, Figure 2 [5197]. Pouch cells are typically packaged in a soft aluminum plastic film. Prismatic cells are rectangular in shape but have a hard shell. The conventional approach to assembly is to combine the cells into modules and then combine the modules into a battery pack, Figure 3. Cells can also be combined directly into packs without modules, a cell-to-pack (CTP) battery system, that can reduce weight, space and cost but increase the challenge of cooling and battery management. Other approaches to battery pack design include cell-to-chassis and cell-to-frame where the battery is integrated into the vehicle chassis or frame.

(Adapted from [5197])
![[schematic]](images/energy/powertrains/batteries/cell_module.avif)
(Source: AZL Aachen)
Cell-to-chassis (CTC) and cell-to-frame (CTF) batteries can be considered to be structural batteries. Structural battery concepts utilize a material such as carbon fiber that can serve as electrode, conductor and load-bearing material and be integrated into the vehicle’s to replace some load bearing structures to achieve significant overall vehicle weight savings [6636][6637][6665].
During charging, electrons are released from the cathode and collected at the anode via an external circuit and Li ions move from the cathode (deintercalate) to the anode (intercalate) via the electrolyte. For an LCO battery:
Cathode: LiyCoO2 ↔ Liy-xCoO2 + xLi+ + xe-(1)
Anode: C6 + xLi+ + xe- ↔ LixC6(2)
During discharging, the reverse reactions occur. Further discussion of Li-ion battery basics can be found elsewhere [5198]. For LCO, only partial deintercalation of Li from the cathode is possible before the cathode is irreversibly damaged and a practical value of x = 0.5. Further discussion of battery charging can be found elsewhere.
###